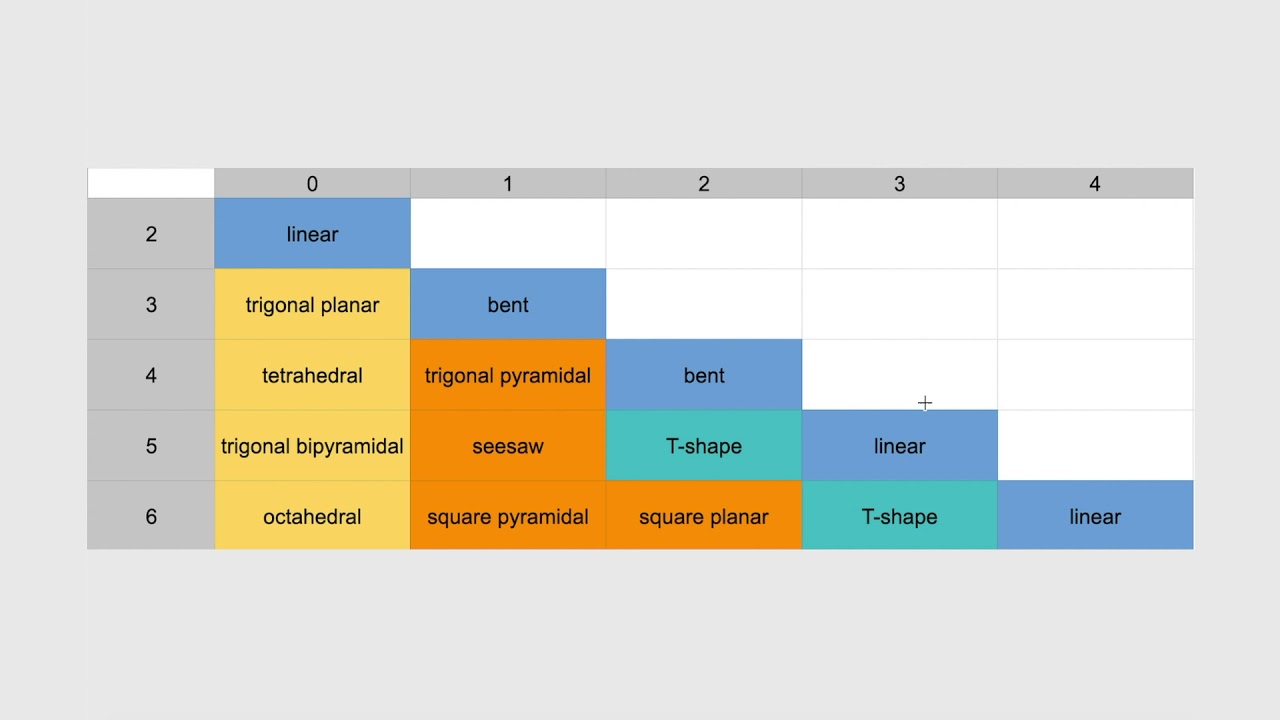
Understanding the Valence Shell Electron Pair Repulsion (VSEPR) chart is more than just a fun exercise in chemistry; it’s a crucial tool for those passionate about molecular shapes and their behaviors. Think of it as a roadmap for predicting how different molecules will shape up in three-dimensional space, directly stemming from electron pair repulsion. This can significantly influence everything from chemical reactivity to physical properties. In a world that’s constantly evolving, grasping the VSEPR chart opens doors to exciting innovations.
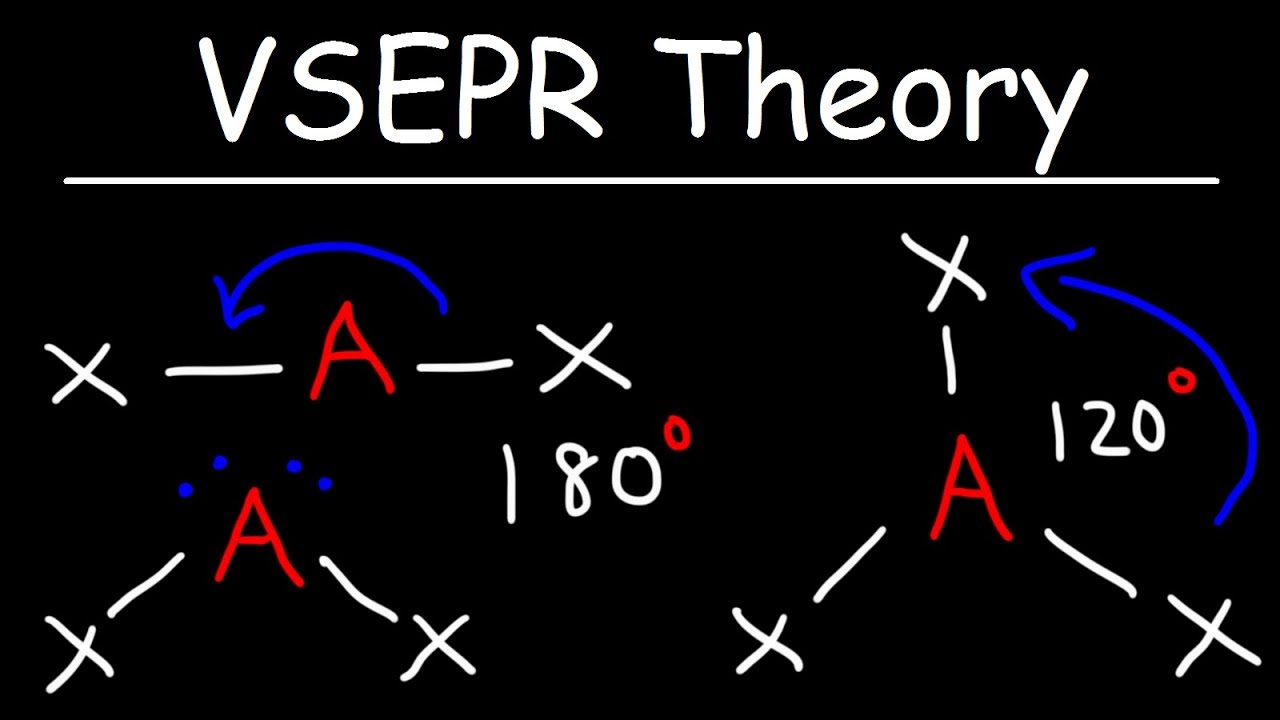
Understanding the VSEPR Chart: A Gateway to Molecular Shape Dynamics
Have you ever wondered why some molecules look the way they do? The VSEPR chart is your key. It organizes various molecular geometries based on the arrangements of electron pairs. With this visual guide, you can easily see how electron pairs repel each other and mold the shape of a molecule. This shapes everything from the carbon dioxide we exhale to the water we drink.
Where does it all lead? The K-12 curriculum usually gives you the basics, but diving deeper reveals real-world applications. From creating new drugs to developing cutting-edge materials for the world’s largest cruise ship, understanding molecular dynamics through the VSEPR chart can make a world of difference. This helps engineers and chemists craft more efficient products and solutions in their fields, sitting at the intersection of science and technology.
Top 5 Molecular Geometries from the VSEPR Chart
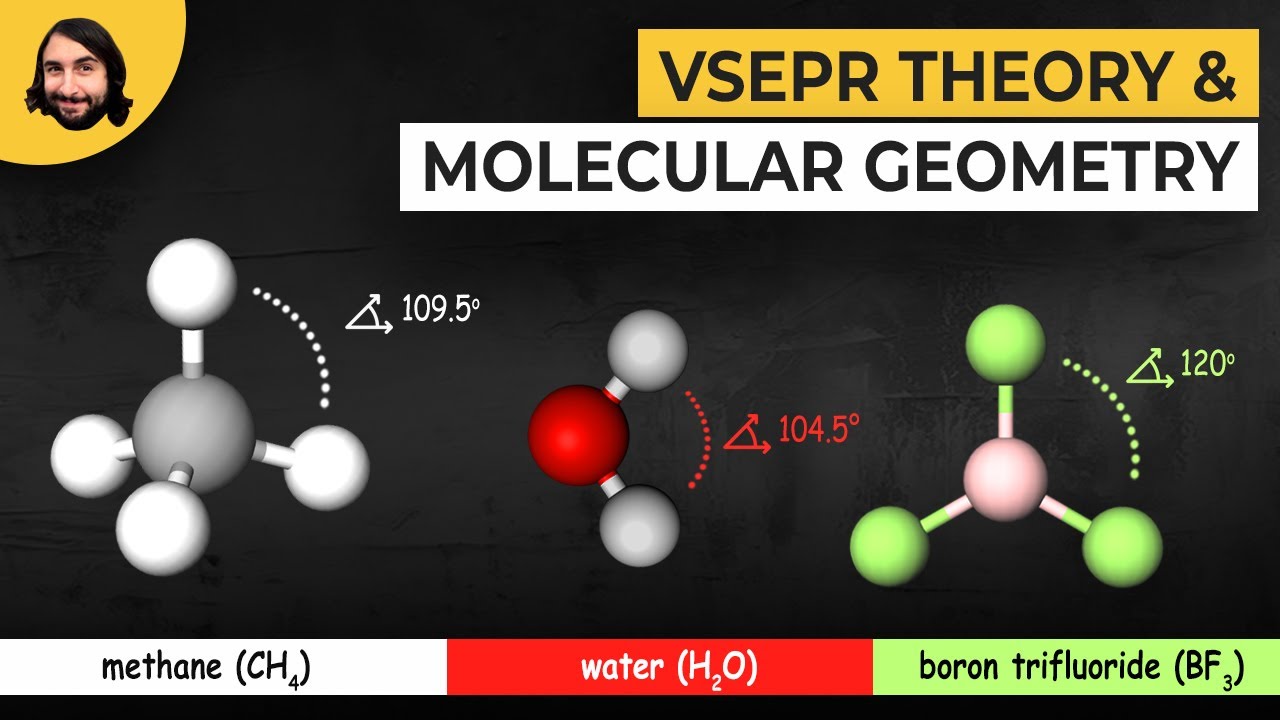
The Influence of Molecular Shape on Chemical Properties
The VSEPR theory doesn’t just teach us about shapes; it helps predict chemical behavior. This insight is instrumental for chemists and engineers alike, as the molecular geometries play a pivotal role in determining various properties.
Polarity and Shape
Understanding molecular shape via the VSEPR chart can reveal why some molecules are polar. Take water (H2O)—its bent shape leads to an uneven distribution of electron density. This characteristic is vital for its unique properties, such as being a solvent for life.
Reactivity Patterns
Geometry influences reactivity. For example, the trigonal bipyramidal structure of phosphorus pentachloride allows for different substitution patterns, impacting its reactivity in synthetic pathways. Grasping this can lead to innovations in chemical synthesis, enhancing efficiency and reducing waste.
Stability and Shape Dynamics
Certain shapes afford greater stability by optimizing electron pair repulsion. The tetrahedral structure minimizes repulsion in saturated hydrocarbons, sustaining stability essential for both structural integrity and efficiency in chemical reactions.
Cultural Perspectives: Molecular Shape Through Artistic Lenses
Interestingly, molecular shapes inspire different forms of art and culture. Take the Society of the Snow, which demonstrates how the intricate structures in snowflakes resemble molecular geometries. These real photos exhibit nature’s elegance and remind us that science isn’t just about formulas and equations—it’s about beauty, too.
Bridging the gap between science and culture enriches our understanding of molecular shapes and dynamics. Art can serve as an engaging medium to communicate scientific principles, making complex concepts more relatable. This blend of interests cultivates curiosity and promotes a deeper appreciation of natural sciences.
Privacy Dynamics in Scientific Research: AOL MyPrivacy Reviews
As innovators navigate the intricacies of molecular structures, privacy and data protection have become crucial focal points. With the rise of digital tools for simulations and data sharing, safeguarding proprietary research is paramount. The AOL MyPrivacy reviews emphasize the importance of maintaining the integrity of scientific inquiry in our modern, technology-driven landscape.
Handling data responsibly fosters trust and ensures that the scientific community can efficiently collaborate and innovate. As we push the boundaries of research and technology, prioritizing privacy will be essential to sustain progress.
Innovative Perspectives on Molecular Geometry
Embracing insights from the VSEPR chart can transform our understanding of molecular interactions and reveal vast possibilities. This awareness extends to various scientific disciplines, including environmental science and material engineering, unlocking doors to sustainable innovations.
Molecular shapes don’t just sit in textbooks; they influence societal narratives and drive technological advancements. The VSEPR chart is a bridge connecting chemistry to broader principles that govern our lives and future. By diving into these molecular dynamics, we enrich ourselves and our understanding of the world around us—demonstrating that even in science, as in life, shapes often define more than just structure.
VSEPR Chart: Insights into Molecular Shape Dynamics
Fun Facts About the VSEPR Chart
Did you know that the VSEPR chart is like a crystal ball for predicting molecular shapes? It’s based on the simple principle that electron groups around a central atom repel each other, leading to distinct molecular geometries. But that’s not all; the way these shapes form can reveal fascinating details about chemical reactions. For instance, just as you’d mix just the right ingredients in a culinary masterpiece while breaking bread with friends, the arrangement of atoms in a molecule is crucial for its properties. The VSEPR chart helps scientists determine these arrangements, almost like Lyrics For lifestyle that define the tune of molecular interactions.
Jumping into the specifics, you’ll find that the classic shapes include linear, trigonal planar, and tetrahedral – sounds like something from a DJ Hernandez set, right? Each geometry corresponds to varying angles and spatial configurations, impacting everything from boiling points to reactivity. And, just as deer blinds help hunters spot their prey, the VSEPR chart helps chemists spot which shapes will perform best in reactions. It’s interesting that something as simple as the number of electron pairs can lead to such a diverse array of shapes – talk about the magic of molecular dynamics!
Interestingly, the history of the VSEPR chart itself is an adventure, evolving as our understanding of chemistry has grown. Think of it like the Worlds Largest cruise ship setting sail—there’s always something new to discover. It’s remarkable how this model enables scientists to visualize complex molecules, much like finding clarity with a tarot card reading through platforms like Findastrologer. For anyone curious or diving into chemistry, understanding the VSEPR chart is like getting your hands on the key to a treasure trove, unlocking intriguing insights into the very fabric of matter. Plus, who knew you could experience it all while contemplating just how diverse life can be, much like the fascinating stories behind plus size Models nude!